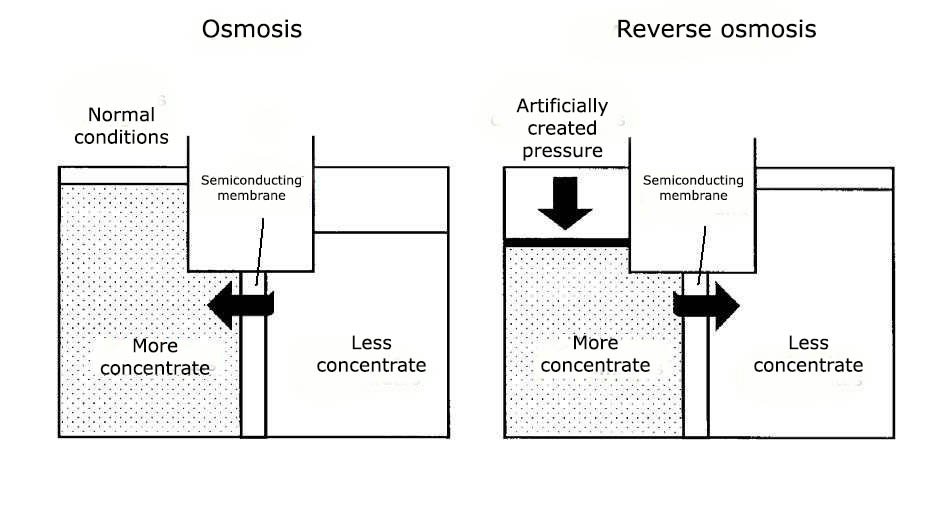
Reverse osmosis is a process in which, at a certain pressure, the solvent (usually water) passes through a semi-permeable membrane from a more concentrated to a less concentrated solution, i.e. in the reverse direction for osmosis. In this case, the membrane skips the solvent, but does not pass some of the dissolved substances in it.
Reverse osmosis is a process in which, at a certain pressure, the solvent (usually water) passes through a semi-permeable membrane from a more concentrated to a less concentrated solution, i.e. in the reverse direction for osmosis. In this case, the membrane skips the solvent, but does not pass some of the dissolved substances in it.
Reverse osmosis has been used since 1970s to purify water, obtain potable water from seawater, and obtain clean water for medicine, industry and other needs. Reverse osmosis can also be used to produce juice concentrates without heating.
Reverse osmosis makes it possible to purify water much more efficiently than the more traditional, mechanical separation of coarse impurities and adsorption of a number of substances. With reverse osmosis, the filtration is at a much finer level - molecular. Even such a system cannot provide 100% purification, but foreign impurities pass through the membranes in the filters in negligible amounts. For most inorganic compounds/elements the filtration is 85% to 98%. Large molecular weight organic matter is almost completely removed. The main gases contained in water - oxygen, hydrogen - do not change their concentration, i.e. the taste of water does not change. The following fact is especially important: bacteria and viruses are large in size, i.e. they are filtered out, and the water is decontaminated.
The output water is very clean and can be used for drinking and cooking even without the need for additional boiling. The minimum content of salts leads to almost complete absence of scale in kettles, dishwashers and washing machines. The properties of filtered water are close to those of melted water. Not prepared at home, received from melting snow, but from ancient glaciers, frozen even when the ecology of the planet was incomparably better.
The process of osmosis is based on the property of water to equalize the level of impurities in solutions separated by a membrane. The holes in this membrane are so small that only water molecules can pass through them. If in one part of such a hypothetical vessel to increase the concentration of impurities, the water will begin to flow there until the density of liquid in both parts of the vessel is equalized.
Reverse osmosis gives the opposite result. In this case, the membrane is used not to equalize the density of the liquid, and in order to collect on one side of it, clean water, and on the other - a solution that is maximally saturated with impurities. That is why this process is called reverse osmosis.